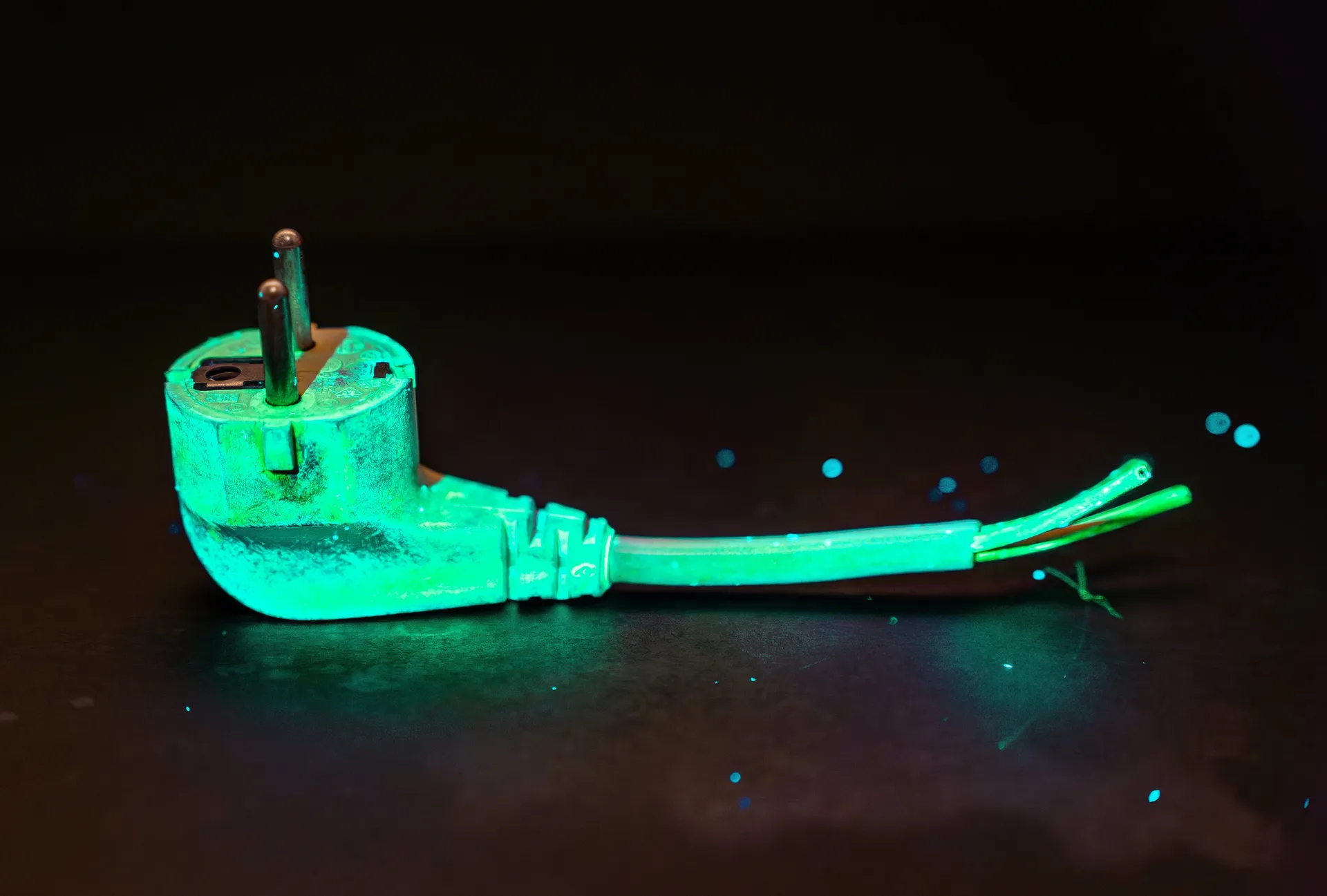
Spray Makes Lead Glow Green in the Environment
Lead is highly toxic, especially to children, and it is pervasive in various materials such as water pipes, paints, glass, electronic components, and ammunition.
Due to activities like mining, coal power plants, or recycling, this heavy metal ends up directly in the environment, posing a particular threat to small children and potentially causing lifelong consequences, including neurological disorders, learning difficulties, and severe physical illnesses. Fortunately, removing this toxin from the environment is relatively straightforward once its presence is identified. The challenge lies in detecting its presence, as it typically requires complex laboratory processes to separate and enrich the element from samples.
Detecting dangerous levels of lead in the environment may become significantly easier in the future. A research group led by Willem L. Noorduin has developed a method in which a sample is sprayed with a chemical, and using a UV lamp, one can immediately determine if lead is present. As reported in the journal “Environmental Science & Technology,” the method correctly identified the presence of lead in experiments with over 50 samples. Moreover, it proved effective even at very low concentrations and with all types of lead compounds. The spray contains Methylammonium bromide, a substance that forms a semiconducting mineral with lead, emitting a green glow under UV light.
The discovery revolves around Perovskites, a class of materials with versatile properties currently under intensive research by various groups and companies. Solar cells based on lead-containing semiconducting Perovskites achieve over 25 percent efficiency. Noorduin and his team originally developed Methylammonium bromide for Perovskite production, and the ability to detect lead in the environment was, according to the research group, a fortunate discovery. The chemical reaction that forms the Perovskite upon contact with lead and spray, even in the presence of water or acid that would typically break down Perovskites, remains somewhat mysterious.